Pharmaceutical Testing
Ensure the quality and safety of pharmaceutical products with our reliable pharma testing services in chennai. API, Formulations, Tablets, injections, Parental.
At Neoscience, we are committed to upholding the safety, efficacy, and quality of pharmaceutical products. Our state-of-the-art laboratories are staffed by a team of highly qualified professionals, including PhD scientists, experienced chemists, and skilled technicians who work tirelessly to deliver accurate and reliable results.
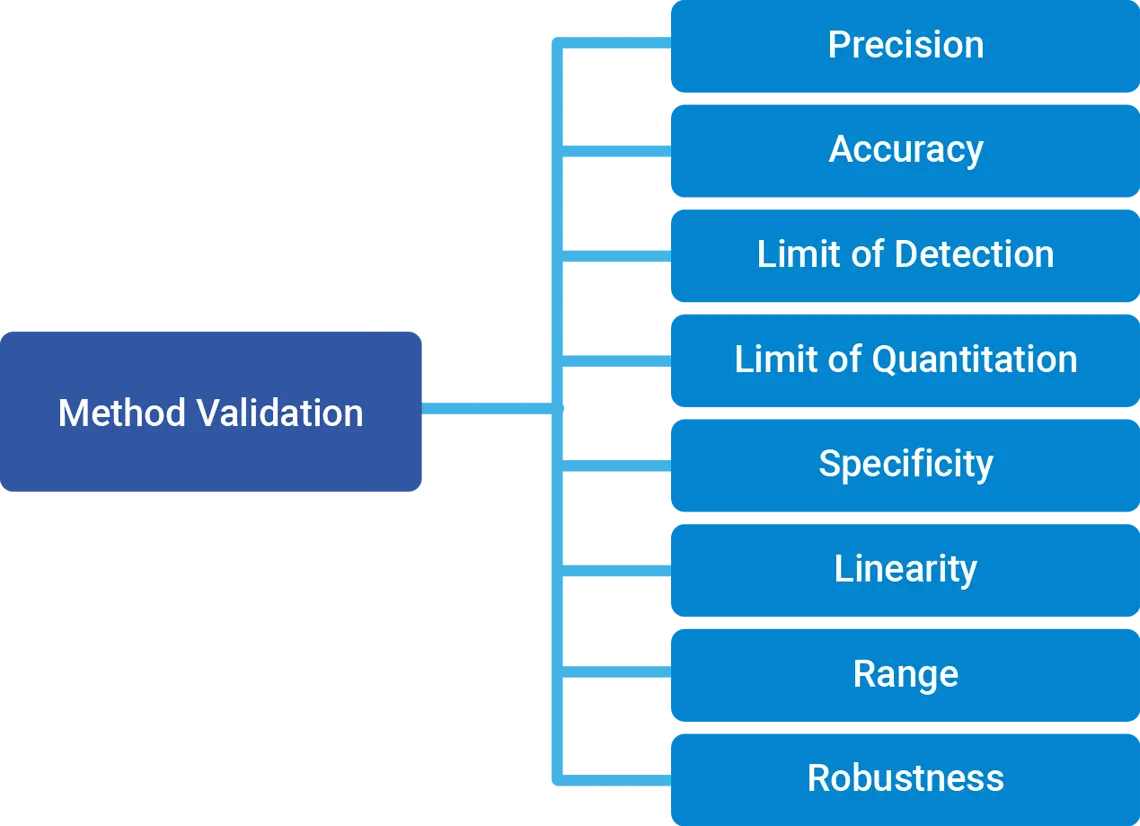
Comprehensive Analytical Services at Neoscience Labs:
Neoscience Labs is dedicated to providing an extensive range of analytical testing services tailored to the pharmaceutical industry. Our commitment to excellence ensures that all testing is conducted with the utmost precision and in compliance with international standards.
Pharmacopeial Testing
International Standards
We conduct pharmacopeial testing based on IP, USP, BP, EP, or JP, ensuring global compliance.
Custom Methods
Utilizing both client-specific and in-house methods, we cater to unique testing requirements.
Heavy Metals and Impurities
Heavy Metals Analysis
Our advanced techniques accurately detect and quantify heavy metal content.
Elemental Impurities
We identify elemental impurities, ensuring the purity of your pharmaceuticals.
Method Development and Quality Control
Method Development and Validation
We develop and validate methods to provide reliable and reproducible results.
Quality Control
Rigorous quality control of raw materials and excipients is performed to maintain product integrity.
Contaminant and Impurity Analysis
Residual Contaminants and Impurities
We proactively manage potential pollutants and impurities, offering a comprehensive range of analytical services to identify and mitigate any issues.
Microbiological and Chemical Analysis
Microbiological Testing
Our labs are equipped to perform sterility testing, microbial identification, and more.
Residual Solvent Analysis
We analyze solvents to ensure they are within safe and acceptable limits.
Stability and Compatibility Studies
Stability Testing
Long-term and accelerated studies are conducted to determine product shelf life.
Extractable and Leachable Studies
We assess the compatibility of packaging materials with the product.
Advanced Microscopy and Spectroscopy
Microscopic Examination
Detailed microscopic analysis provides insights into the physical characteristics of samples.
Spectroscopy
Our spectroscopic methods offer precise identification and quantification of compounds.
Regulatory Compliance Testing
Limit Tests
Standardized protocols are followed for limit tests to ensure regulatory compliance.
Cleaning Validation
We validate cleaning processes to prevent cross-contamination.
Environmental and Safety Testing
Clean Room Monitoring
Environmental monitoring in clean rooms is conducted to maintain controlled conditions.
Disinfectant Efficacy
Chemical disinfectant testing and cleaning studies are performed to ensure a sterile environment.
Bioanalytical Services
Endotoxin and Microorganism Testing
We use the LAL/gel clot method for bacterial endotoxins testing and conduct specific microorganism testing.
Bioburden Testing
Our bioburden testing services evaluate the microbial load on pharmaceutical products.
Validation and Efficacy Studies
Method Validations
MLT, BET, Bioburden, and sterility method validations are performed to confirm testing accuracy.
Efficacy Testing
Preservative efficacy and antimicrobial effectiveness testing are conducted to ensure product safety.
Our Expert Team:
Experienced Scientists
Our team comprises industry-leading experts with extensive experience in pharmaceutical testing and analysis.
Dedicated Chemists
Our chemists specialize in developing and validating analytical methods tailored to your specific needs.
Skilled Technicians
Our technicians are trained in the latest laboratory techniques and operate under strict quality control measures.
Advanced Infrastructure:
Modern Laboratories
Our facilities are equipped with cutting-edge instruments for a wide array of tests and analyses.
Innovative Technology
We utilize the latest advancements in LCMS/MS, GCMS/MS, ICPMS, HPLC, and more to ensure high precision.
Robust Quality Systems
Our laboratories operate under cGMP conditions, with rigorous quality systems in place to ensure compliance with all regulatory standards.
Comprehensive Testing Capabilities
Raw Material and Finished Product Testing
We perform in-depth analysis and quality control tests to verify the composition and ensure the final product meets all specifications.
Stability and Method Validation
Our stability studies and method validation processes are designed to be robust and reproducible, providing you with confidence in your product's longevity and our testing accuracy.
Regulatory Compliance and Methodology:
21-CFR Compliance
We strictly adhere to CGMP regulations and ensure our methods meet the requirements of the United States, Europe, the Rest of the World, and India.
Method Development and Validation
Our method development is meticulous, and our validation processes follow ICH (Q2A, Q2B) and FDA guidelines to the letter.
High-Performance Testing Services:
Fast and Reliable Information
Our high-performance teams provide you with rapid and accurate data, enabling data-driven decisions for your business.
Flexible Solutions
We offer tailored solutions to meet your business needs, whether you require full-time support or assistance with managing a heavy workload.
Contact us today
Ensure the highest standards of safety and quality with our expert testing services. Contact us today for unparalleled Food and Feed Testing, precise Pharmaceuticals Testing, and reliable Analytical Services. Your peace of mind starts with our accuracy and expertise.
Subscribe to our newsletter
Stay ahead in the world of scientific advancements and innovation. Join our mailing list to receive the latest news, updates and exclusive offers from Neoscience Labs directly to your inbox.